Intriguing Geometries Trigonal Pyramidal In Chemistry Bond Angle The Best Porn Website
One example of a molecule with a trigonal pyramidal geometry is ammonia (nh 3). The three atoms and the lone pair form a pyramid shape with the central atom at the apex. This type of molecular geometry occurs when there are three atoms or ions bonded to a central atom and one lone pair of electrons on the central atom.
Trigonal Pyramidal Bond Angle The Best Porn Website
Trigonal pyramidal geometry typically involves a central atom bonded to three other atoms, forming a pyramid shape with a lone pair of electrons at the apex. The trigonal pyramidal geometry exists when there are 3 bonds and 1 lone pair. In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base.
- Jp Morgan Recruiting Process
- Craigslist Shelbyville
- Manuela Escobar A Life Beyond Notoriety
- Where Has Rita Panahi Been Uncovering Her Journey And Achievements
- Meet Daniela Bobadilla A Closer Look At Her Life And Career
You will learn about the more common molecular geometries:
This is seen in ammonia (right). Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. This guide highlights molecular geometry, bond angles, hybridization, and electron pair arrangements, providing clear insights into their differences. Learn how bond angles, electron pair arrangements, and hybridization distinguish these shapes, essential for understanding vsepr theory and molecular structures in chemistry.
Trigonal pyramidal geometry arises when the central atom is connected to three other atoms and contains a single lone pair of electrons. Discover the secrets of the trigonal pyramidal angle and its impact on molecular geometry. When all three atoms at the corners are identical the molecule belongs to point group c 3v. The world of chemistry is filled with fascinating shapes and configurations that govern the behavior and properties of molecules.

Trigonal pyramidal molecular geometry Wikipedia
Trigonal pyramidal and trigonal planar are two common molecular geometries in chemistry.
Perfect for students and enthusiasts exploring chemical. Discover the key differences between trigonal planar and trigonal pyramidal molecular geometries in this quick guide. Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Among these, the trigonal pyramidal geometry stands out for its unique structure and significant role in determining molecular characteristics.
The single lone pair sits on top of the molecule where the 4th bond in the tetrahedral structure is. Trigonal pyramidal (ax3e) this is an example of a trigonal pyramidal molecule. In general, when the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. These molecules have four atoms in the shape of a pyramid with a base in the shape of a triangle.

Trigonal Pyramidal Structure
Sp 3 hybridization occurs at the centre atom of molecules with tetrahedral electron pair geometries.
This configuration is commonly seen in molecules like ammonia (nh₃). Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. The bond angle for trigonal pyramidal geometries is less than `109.5^@` due to the additional repulsion from the lone pair. This guide clarifies how these structures impact molecular shape, polarity, and reactivity, helping you understand complex chemistry topics with ease.
Discover the 9 key differences between trigonal planar and pyramidal molecular geometries. It’s like a tetrahedral molecule, except that one of the atoms is replaced with a lone pair of electrons. Molecules with an tetrahedral electron pair geometries have sp 3 hybridization at the central atom. Trigonal planar geometry occurs when the central atom is connected to three other atoms without any lone pairs of electrons.
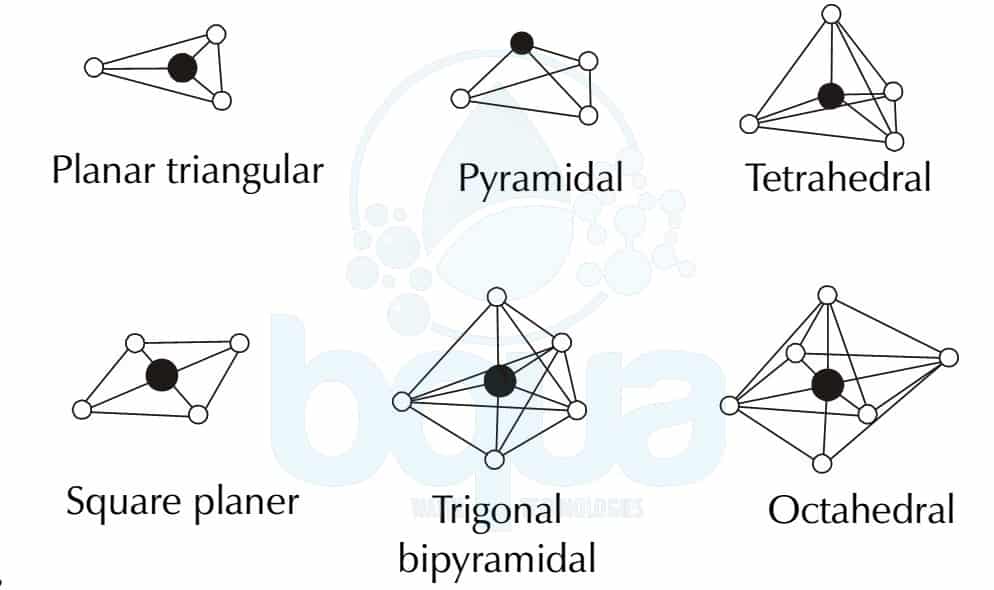
Trigonal Pyramidal Molecular Geometry Archives BQUA
In trigonal pyramidal geometry, the central atom is surrounded by three other atoms and one lone pair of electrons.
This results in a shape that resembles a pyramid with a triangular base, but with one less atom than the tetrahedral shape. This article delves into the bond angles, shape, and properties of trigonal pyramidal molecules, highlighting their significance in chemical structures, hybridization, and molecular polarity, essential for understanding complex chemical interactions and reactions. In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule. Master essential concepts like bond angles, electron pair arrangements, and hybridization.
Trigonal pyramidal and trigonal planar are two common molecular geometries in chemistry. Discover the 12+ key features to easily distinguish trigonal planar from pyramidal structures. In a trigonal pyramidal geometry, the central atom is surrounded by three other atoms and one lone pair of electrons. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules.

Trigonal Pyramidal Bond Angle The Best Porn Website